
Chemistry, 19.03.2020 21:13 YouKnowGucci
Which of the following statements about the reaction quotient, Q is false? a. The value of Q can be used to predict equilibrium concentrations. b. It has the same expression as Kc. c. Its value is calculated using nonequilibrium concentrations. d. If Q > Kc, the reaction must move to equilibrium by forming more reactants. e. If Q < Kc, the reaction must move to equilibrium by forming more products.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Which of the following statements about the reaction quotient, Q is false? a. The value of Q can be...
Questions in other subjects:




Mathematics, 09.11.2019 17:31

Social Studies, 09.11.2019 17:31

English, 09.11.2019 17:31




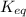
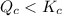 , reaction will move in forward direction and concentration of products will increase.
, reaction will move in forward direction and concentration of products will increase.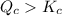 , reaction will move in backward direction and concentration of reactant will increase.
, reaction will move in backward direction and concentration of reactant will increase.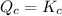 reaction is at equilibrium.
reaction is at equilibrium.


