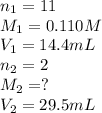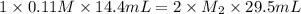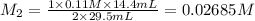Chemistry, 19.03.2020 20:29 adhitrfbvg
An aqueous solution of barium hydroxide is standardized by titration with a 0.110 M solution of hydrochloric acid. If 29.5 mL of base are required to neutralize 14.4 mL of the acid, what is the molarity of the barium hydroxide solution

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2



You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.110 M solution of hydr...
Questions in other subjects:

English, 04.08.2019 20:30



Physics, 04.08.2019 20:30

History, 04.08.2019 20:30

Mathematics, 04.08.2019 20:30

History, 04.08.2019 20:30

Physics, 04.08.2019 20:30

Geography, 04.08.2019 20:30

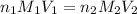
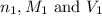 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 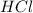
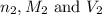 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 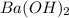 mm.
mm.