
Chemistry, 19.03.2020 20:33 tfaulk2884
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) In an experiment, 201 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 732torr. How many grams of Zn have been consumed? The vapor pressure of water at 27 °C is 26.74 torr.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, babygirl2984
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1


Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3
You know the right answer?
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) I...
Questions in other subjects:

Biology, 08.12.2020 22:10

Mathematics, 08.12.2020 22:10

Mathematics, 08.12.2020 22:10



Mathematics, 08.12.2020 22:20

Biology, 08.12.2020 22:20

English, 08.12.2020 22:20

English, 08.12.2020 22:20

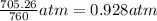
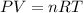
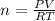
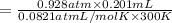
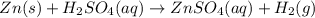
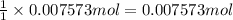 of zinc
of zinc


