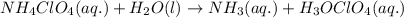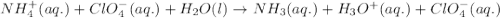Chemistry, 19.03.2020 20:29 lalaokawami0912
Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium perchlorate is dissolved in water. (Use H3O instead of H .)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, sabahfayaskhan
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1


Chemistry, 22.06.2019 21:00, thebasedgodchri
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammon...
Questions in other subjects:


Social Studies, 04.07.2019 08:30


History, 04.07.2019 08:30

History, 04.07.2019 08:30


Chemistry, 04.07.2019 08:30


Business, 04.07.2019 08:30

Spanish, 04.07.2019 08:30

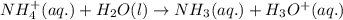
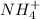 and
and 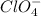 ions.
ions. 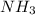 .
. 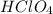 .
.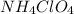 ,
, 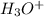 .
.