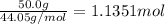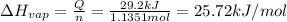29.2 kJ of heat is required to convert an 50.0 g sample of
acetaldehyde from the liquid to gas...

Chemistry, 19.03.2020 20:37 rexerlkman4145
29.2 kJ of heat is required to convert an 50.0 g sample of
acetaldehyde from the liquid to gas phase. The molecular
weight of acetaldehyde is 44.05 g/mol. What is the heat of
vaporization of acetaldehyde in kJ/mol?
kJ/mol

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 06:30, amylumey2005
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 07:30, sweetLips230
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 22.10.2020 03:01


Mathematics, 22.10.2020 03:01

Advanced Placement (AP), 22.10.2020 03:01


History, 22.10.2020 03:01

Chemistry, 22.10.2020 03:01

Mathematics, 22.10.2020 03:01