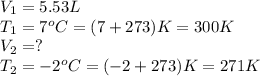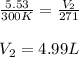An arctic weather balloon is filled with 5.53L of helium gas inside a prep shed. The temperature inside the shed is 7.°C. The balloon is then taken outside, where the temperature is −2.°C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1atm. Be sure your answer has the correct number of significant digits. L

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1
You know the right answer?
An arctic weather balloon is filled with 5.53L of helium gas inside a prep shed. The temperature ins...
Questions in other subjects:

Computers and Technology, 13.07.2019 17:00

Health, 13.07.2019 17:00




Biology, 13.07.2019 17:00




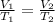
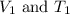 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.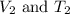 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.