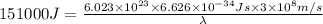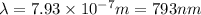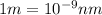Chemistry, 19.03.2020 17:17 christophergaudette0
It takes to break an iodine-iodine single bond. Calculate the maximum wavelength of light for which an iodine-iodine single bond could be broken by absorbing a single photon. Be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, moneykey
Hannah is writing a report on how albedo affects the global climate. she’s proofreading her passage for any factual errors. which sentence must hannah correct before submitting her report? earth receives energy from the sun. this energy drives many of the processes on earth, including its climate. some part of this energy is reflected by earth’s surface. we use the term albedo to describe the reflected energy. albedo of an object is the ratio of the reflected radiation to the total radiation reaching the object. a value of 0 means no energy is absorbed by the object, whereas a value of 1 means that all of the energy is absorbed. in this way, the albedo of an object can influence earth’s atmospheric temperature.
Answers: 1

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

You know the right answer?
It takes to break an iodine-iodine single bond. Calculate the maximum wavelength of light for which...
Questions in other subjects:

Mathematics, 10.02.2021 19:50

Mathematics, 10.02.2021 19:50




Mathematics, 10.02.2021 19:50

Arts, 10.02.2021 19:50

Mathematics, 10.02.2021 19:50


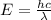
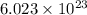
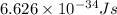
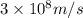
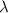 = wavelength of light = ?
= wavelength of light = ?