
Chemistry, 19.03.2020 17:10 studybuddy0203
Predict the effect of increasing the container volume on the amounts of each reactant and product in the following reactions: (a) CH3OH(l) <--> CH3OH(g) (b) CH4(g) + NH3(g) <--> HCN(g) + 3H2(g)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
Predict the effect of increasing the container volume on the amounts of each reactant and product in...
Questions in other subjects:

Health, 05.05.2020 06:21

English, 05.05.2020 06:21

History, 05.05.2020 06:21


Arts, 05.05.2020 06:21


English, 05.05.2020 06:21





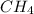 and 1 mol of
and 1 mol of 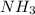 ) and in the right side we will have 4 moles (1 mol of
) and in the right side we will have 4 moles (1 mol of 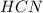 and 3 mol of
and 3 mol of 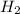 ). Therefore, the reaction will move to the right side.
). Therefore, the reaction will move to the right side.


