Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it ob...

Chemistry, 19.03.2020 17:16 jadielmatmat
Consider this reaction:
2H3PO4(aq)→ P2O5(aq)+ 3H2O
At a certain temperature it obeys this rate law. rate
rate= (46.6M^-1. s^-1) [H3PO4]^2
Suppose a vessel contains H3PO4 at a concentration of 0.660M. Calculate how long it takes for the concentration of H3PO$ to decrease to 20% to its natural value. You may assume no other reaction is important. Round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 23:00, edgar504xx
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 20.11.2019 21:31


Mathematics, 20.11.2019 21:31

Biology, 20.11.2019 21:31






![Rate=k[H_3PO_4]^2](/tpl/images/0554/2402/79104.png)
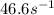
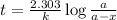
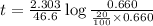
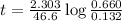
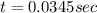
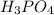 to decrease to 20% to its natural value is 0.0345 sec
to decrease to 20% to its natural value is 0.0345 sec

