Chemistry, 19.03.2020 09:31 priscillarios30
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g) Calculate the equilibrium concentrations of reactant and products when 0.372 moles of HI are introduced into a 1.00 L vessel at 698 K. [HI] = M [H2] = M [I2] = M

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g)...
Questions in other subjects:

Mathematics, 07.07.2021 05:10

History, 07.07.2021 05:10

Physics, 07.07.2021 05:10

Business, 07.07.2021 05:10

Mathematics, 07.07.2021 05:10

History, 07.07.2021 05:10



Mathematics, 07.07.2021 05:10

History, 07.07.2021 05:10

![[HI]=(0.372-2x) M =(0.372-2\times 0.03935)M =0.2933 M](/tpl/images/0553/9717/216fb.png)
![[H_2]=x = 0.03935 M](/tpl/images/0553/9717/0d5d5.png)
![[I_2]=x = 0.03935 M](/tpl/images/0553/9717/d2ad6.png)
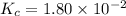
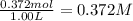
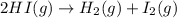
![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0553/9717/ef85e.png)