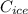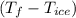2. A 10.0 g ice cube, initially at 0.0ºC, is melted in 100.0 g of water that was initially 20.0ºC. After the ice has melted, the equilibrium temperature is 10.93ºC. Show your work!
a. Calculate the total heat (in J) lost by the water (specific heat for water is 4.184 J/g°C).
b. Calculate the heat gained by the ice cube after it melts (specific heat for ice is 2.093 J/g°C).
c. Calculate the heat it took to melt the ice. (It takes 334.0 J/g of heat energy to melt ice). qmelt = mice ΔHmelt

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 05:00, daytonalive6511
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3

Chemistry, 23.06.2019 09:00, floressavanna15
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 09:30, crawford184232323234
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
2. A 10.0 g ice cube, initially at 0.0ºC, is melted in 100.0 g of water that was initially 20.0ºC. A...
Questions in other subjects:

Biology, 07.05.2021 01:00


Mathematics, 07.05.2021 01:00





Biology, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

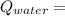 3.8 KJ
3.8 KJ 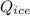 = 228.76 J
= 228.76 J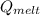 = 3340 J
= 3340 J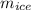 = 10 gm
= 10 gm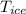 = 0 °c
= 0 °c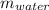 = 100 gm
= 100 gm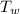 = 20 °c
= 20 °c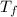 = 10.93 °c
= 10.93 °c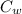 (
(