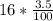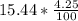3.
A student uses a solution that contains 16 grams of water to conduct an evaporation
exp...

3.
A student uses a solution that contains 16 grams of water to conduct an evaporation
experiment.
At the end of one hour, the amount of water in the solution has decreased
by 3.5%.
. At the end of two hours, the amount of water in the solution has decreased
by another 4.25%.
Which calculations can be used to determine the amount of water, in grams, remaining
in the solution at the end of the second hour?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2
You know the right answer?
Questions in other subjects:

History, 28.07.2019 12:00



Mathematics, 28.07.2019 12:00


History, 28.07.2019 12:00

English, 28.07.2019 12:00