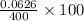A 0.400-g sample of toothpaste was boiled with a 50-mL solution containing a citrate buffer and NaCl to extract a fluoride ion. After cooling, the solution was diluted to exactly 100 mL. The potential of ISE with Ag-AgCl (sat) reference electrode in a 25.0-mL aliquote of the sample was found to be -0.1823 V. Addition of 5.0 mL of a solution containing 0.00107 mg F-/mL caused the potential to change to -0.2446 V. Calculate the percentage of F- in the sample.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
A 0.400-g sample of toothpaste was boiled with a 50-mL solution containing a citrate buffer and NaCl...
Questions in other subjects:

Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10


Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10

Computers and Technology, 31.07.2019 01:10

 = 0.0053 mg)
= 0.0053 mg)  potential will change into -0.2446 V. Hence, potential created by 0.00535 mg
potential will change into -0.2446 V. Hence, potential created by 0.00535 mg  )
)