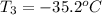Chemistry, 19.03.2020 07:42 kcceff4085
A food product is being frozen in a system capable of removing 6000 kJ of thermal energy. The product has a specifi c heat of 4 kJ/(kg C) above the freezing temperature of 2 C, the latent heat of fusion equals 275 kJ/kg, and the frozen product has a specifi c heat of 2.5 kJ/(kg C) below 2 C. If 10 kg of product enters the system at 10C, determine the exit temperature of the product..

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2



Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
A food product is being frozen in a system capable of removing 6000 kJ of thermal energy. The produc...
Questions in other subjects:

History, 18.12.2019 17:31

English, 18.12.2019 17:31

Biology, 18.12.2019 17:31







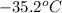
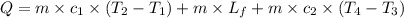
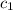 = specific heat of liquid =
= specific heat of liquid = 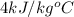
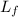 = latent heat of fusion =
= latent heat of fusion = 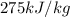
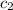 = specific heat of frozen =
= specific heat of frozen = 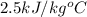
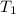 = initial temperature of liquid =
= initial temperature of liquid = 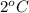
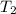 = final temperature of liquid =
= final temperature of liquid = 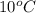
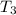 = initial temperature of frozen = ?
= initial temperature of frozen = ?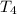 = final temperature of frozen =
= final temperature of frozen = ![6000kJ=[15kg\times 4kJ/kg^oC\times (10-2)^oC]+[15kg\times 275kJ/kg]+[15kg\times 2.5kJ/kg^oC\times (2-T_3)^oC]](/tpl/images/0553/7186/e8cbe.png)