
Chemistry, 19.03.2020 06:31 nikejenkins18
Consider a rigid, insulated box containing 0.400 mole of he(g) at 20.08c and 1.00 atm in one compartment and 0.600 mole of n2(g) at 100.08c and 2.00 atm in the other compartment. these compartments are connected by a partition that transmits heat. what is the final tempera-ture in the box at thermal equilibrium? [for he(g), cv 5 12.5 j k 21mol 21 ; for n2(g), cv 5 20.7 j k 21mol 21

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 07:30, shealynh52
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m. a. calculate the kc value for the a-protein binding reaction. b. calculate the kc value for the b-protein binding reaction. c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
You know the right answer?
Consider a rigid, insulated box containing 0.400 mole of he(g) at 20.08c and 1.00 atm in one compart...
Questions in other subjects:






Mathematics, 10.04.2020 01:00



English, 10.04.2020 01:00

Mathematics, 10.04.2020 01:00

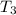 = 341.08 K
= 341.08 K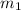 = 0.4 mole
= 0.4 mole = 20.08 °c = 293.08 K
= 20.08 °c = 293.08 K = 1 atm = 101.325 K pa
= 1 atm = 101.325 K pa = 0.6 mole
= 0.6 mole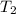 = 373.08 K
= 373.08 K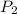 = 2 atm = 202 K pa
= 2 atm = 202 K pa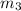 =
= 

