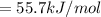Chemistry, 19.03.2020 05:55 bryanmcmillianjr
Consider the Gibbs energies at 25 ∘ C. Substance Δ G ∘ f ( kJ ⋅ mol − 1 ) Ag + ( aq ) 77.1 Cl − ( aq ) − 131.2 AgCl ( s ) − 109.8 Br − ( aq ) − 104.0 AgBr ( s ) − 96.9 (a) Calculate Δ G ∘ rxn for the dissolution of AgCl ( s ) .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Consider the Gibbs energies at 25 ∘ C. Substance Δ G ∘ f ( kJ ⋅ mol − 1 ) Ag + ( aq ) 77.1 Cl − ( aq...
Questions in other subjects:

History, 27.04.2021 01:00

English, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Arts, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00

Mathematics, 27.04.2021 01:00

Physics, 27.04.2021 01:00


Mathematics, 27.04.2021 01:00

![\Delta G_{rxn}^o=\sum[\Delta G^o_{f}]_{products}-\sum[\Delta G^o_{f}]_{reactants}](/tpl/images/0553/6074/2b279.png)
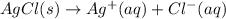
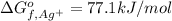
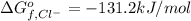
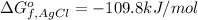
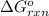 :
:![\Delta G_{rxn}^o=[77.1 kJ/mol+(-131.2 kJ/mol)]-[-109.8 kJ/mol]](/tpl/images/0553/6074/68588.png)