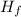Chemistry, 19.03.2020 05:38 Marshmallow9174
Given that the heat of fusion of water is +6.02 kJ/mol that the heat capacity of H2O(l)H2O(l) is 75.2 J/mol⋅KJ/mol⋅K and that the heat capacity of H2O(s)H2O(s) is 37.7 J/mol⋅KJ/mol⋅K, calculate the heat of fusion of water at -15 ∘C∘C.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, jasmineharris121
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1
You know the right answer?
Given that the heat of fusion of water is +6.02 kJ/mol that the heat capacity of H2O(l)H2O(l) is 75....
Questions in other subjects:

Mathematics, 08.12.2020 01:20


Mathematics, 08.12.2020 01:20

Health, 08.12.2020 01:20

Social Studies, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

Chemistry, 08.12.2020 01:20

Mathematics, 08.12.2020 01:20

 ·(T₂ - T₁)
·(T₂ - T₁)