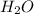The following reaction has Kc > 1: Ni(H2O)62+(aq) + 6 NH3(aq) → Ni(NH3)62+(aq) + 6 H2O(l) Based on this information, what can you conclude about the strength of NH3 as a Lewis acid/base? Select all that apply. Group of answer choices NH3 is a stronger Lewis base than H2O NH3 is a weaker Lewis base than H2O NH3 is a stronger Lewis acid than H2O NH3 is a weaker Lewis acid than H2O

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
The following reaction has Kc > 1: Ni(H2O)62+(aq) + 6 NH3(aq) → Ni(NH3)62+(aq) + 6 H2O(l) Based o...
Questions in other subjects:


History, 20.09.2019 12:30

Mathematics, 20.09.2019 12:30


Business, 20.09.2019 12:30

Social Studies, 20.09.2019 12:30



Physics, 20.09.2019 12:30

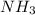 is a stronger Lewis base than
is a stronger Lewis base than