
Chemistry, 19.03.2020 04:08 maskythegamer
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in the atmosphere occur mainly in regions with large automobile or power plant emissions. The equilibrium constant for the reaction of N2 and 02 to give NO is very small. The reaction is, however, highly endothermic, with a heat of reaction equal to +180 kJ (Equation 7). N2(g) + O2(g) 180 kJ 2NO(g) Equation 7 +
(a) Use LeChâtelier's Principle to explain why the concentration of NO at equilibrium increases when the reaction takes place at high temperatures.
(b) Use LeChâtelier's Principle to predict whether the concentration of NO at equilibrium should increase when the reaction takes place at high pressures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 19:20, johnkings140
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Although both N2 and 02 are naturally present in the air we breathe, high levels of NO and NO2 in th...
Questions in other subjects:

Biology, 02.07.2019 19:00



Mathematics, 02.07.2019 19:00

History, 02.07.2019 19:00





Mathematics, 02.07.2019 19:00

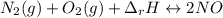
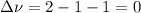
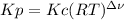
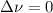 ,
, 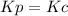 , so no effect in concentration is due to the pressure.
, so no effect in concentration is due to the pressure.

