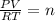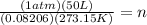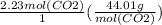Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
I need help with this question...
The equation for methane is ( CH4(g) + 2O2(g) --> 2...
The equation for methane is ( CH4(g) + 2O2(g) --> 2...
Questions in other subjects:



History, 22.07.2019 13:30

History, 22.07.2019 13:30



Social Studies, 22.07.2019 13:30


Mathematics, 22.07.2019 13:40