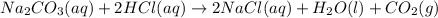A student combined two colorless aqueous solutions. One of the solutions contained Na2CO3 as the solute and the other contained HCl . The chemical reaction that took place is represented by the equation above. What experimental result would be evidence that a chemical reaction took place when the solutions were combined

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2
You know the right answer?
A student combined two colorless aqueous solutions. One of the solutions contained Na2CO3 as the sol...
Questions in other subjects:

English, 17.11.2019 07:31





Mathematics, 17.11.2019 07:31

Mathematics, 17.11.2019 07:31



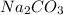 and
and 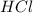 as a solute will be combined, the reaction will be as follows,
as a solute will be combined, the reaction will be as follows,