
Chemistry, 19.03.2020 01:31 22katelynfrankouqqrb
One way to represent this equilibrium is: N2O4(g)2NO2(g) Indicate whether each of the following statements is true, T, or false, F. AT EQUILIBRIUM we can say that: 1. The concentration of NO2 is equal to the concentration of N2O4. 2. The rate of the dissociation of N2O4 is equal to the rate of formation of N2O4. 3. The rate constant for the forward reaction is equal to the rate constant of the reverse reaction. 4. The concentration of NO2 divided by the concentration of N2O4 is equal to a constant.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

You know the right answer?
One way to represent this equilibrium is: N2O4(g)2NO2(g) Indicate whether each of the following stat...
Questions in other subjects:

Geography, 23.08.2019 06:30

Business, 23.08.2019 06:30



Mathematics, 23.08.2019 06:30



English, 23.08.2019 06:30


 is equal to the concentration of
is equal to the concentration of 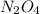 : False
: False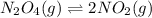
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0553/2027/271f5.png)


