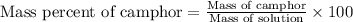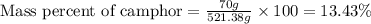Chemistry, 19.03.2020 01:36 briseisr20
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the camphor tree. Assume you dissolve 70.0 g of camphor (C10H16O) in 575 mL of ethanol, C2H5OH. Calculate the molarity, molality, mole fraction, and weight percentage of camphor in this solution. (The density of ethanol is 0.785 g/mL.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

You know the right answer?
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the...
Questions in other subjects:

Physics, 26.08.2019 00:30






History, 26.08.2019 00:30

Social Studies, 26.08.2019 00:30

History, 26.08.2019 00:30

Mathematics, 26.08.2019 00:30

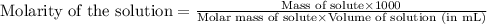
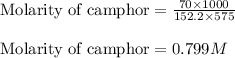
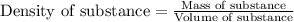
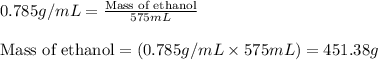
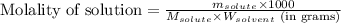
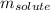 = Given mass of solute (camphor) = 70 g
= Given mass of solute (camphor) = 70 g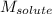 = Molar mass of solute (camphor) = 152.2 g/mol
= Molar mass of solute (camphor) = 152.2 g/mol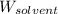 = Mass of solvent (ethanol) = 451.38 g
= Mass of solvent (ethanol) = 451.38 g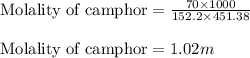
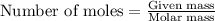 .....(1)
.....(1)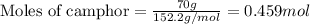
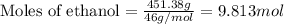
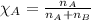
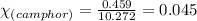 \
\