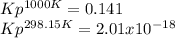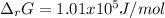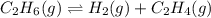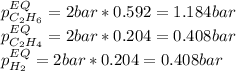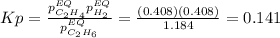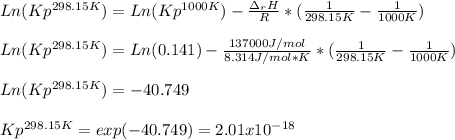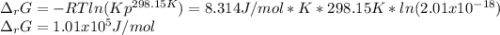Consider the equilibrium
At 1000 K and a const. total pressure of 2 bar, C2H6 is introd...

Consider the equilibrium
At 1000 K and a const. total pressure of 2 bar, C2H6 is introduced into a reaction vessel. the total pressure is held const. at 2 bar and at equilibrium the composition of the mixture in mole percent is H2(g): 20.4%, C2H4 (g): 20.4%, and C2H6 (g): 59.2%.
Calculate Kp at 1000 K
if ΔH of reaction = 137 kJ/mol, calculate the value of Kp at 298.15K
Calculate ΔG of reaction for this reaction at 298.15 K

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Questions in other subjects:


Social Studies, 01.09.2019 05:20

Physics, 01.09.2019 05:20

Social Studies, 01.09.2019 05:20



History, 01.09.2019 05:20


Social Studies, 01.09.2019 05:20