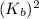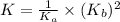Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3



Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Consider the reaction: N2(g) 2 O2(g)N2O4(g) Write the equilibrium constant for this reaction in term...
Questions in other subjects:

Social Studies, 18.02.2021 16:40

Geography, 18.02.2021 16:40



History, 18.02.2021 16:40

Arts, 18.02.2021 16:40


Mathematics, 18.02.2021 16:40


Mathematics, 18.02.2021 16:40

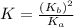
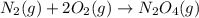 ;
; 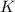
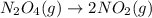 ;
; 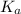
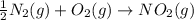 ;
; 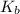
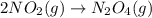 ;
; 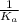
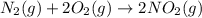 ;
;