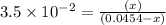Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10−2 If you start with 25.0 mLmL of a 0.909 MM solution of NaIO4NaIO4, and then dilute it with water to 500.0 mLmL, what is the concentration of H4IO−6H4IO6− at equilibrium?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1
You know the right answer?
Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10...
Questions in other subjects:


Business, 03.08.2019 05:30




History, 03.08.2019 05:30


Computers and Technology, 03.08.2019 05:30

Computers and Technology, 03.08.2019 05:30

Mathematics, 03.08.2019 05:30

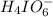 at equilibrium is, 0.00154 M
at equilibrium is, 0.00154 M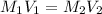
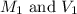 are the initial molarity and volume of
are the initial molarity and volume of 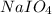 .
.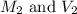 are the final molarity and volume of diluted
are the final molarity and volume of diluted 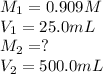
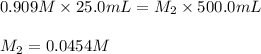
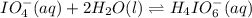
![K_c=\frac{[H_4IO_6^-]}{[IO_4^-]}](/tpl/images/0553/1589/ffca5.png)