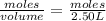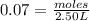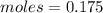Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:40, vanessa23272
How can you increase the ability of a gas to dissolve in a liquids?
Answers: 1


You know the right answer?
10. At a certain temperature, Kc equals 1.4 × 102 for the reaction:2 CO(g) + O2(g) ⇌ 2 CO2(g). If a...
Questions in other subjects:


Mathematics, 03.03.2021 14:30

History, 03.03.2021 14:30


Mathematics, 03.03.2021 14:30

English, 03.03.2021 14:30

Biology, 03.03.2021 14:30

English, 03.03.2021 14:30


Mathematics, 03.03.2021 14:30

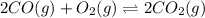
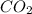 =
= 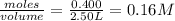
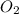 =
= 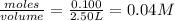
![K_c=\frac{[CO_2]^2}{[CO]^2\times [O_2]}](/tpl/images/0553/0997/662e3.png)
![1.4\times 10^2=\frac{(0.16)^2}{[CO]^2\times 0.04}](/tpl/images/0553/0997/5df84.png)
![[CO]=0.07M](/tpl/images/0553/0997/06724.png)
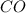 =
=