
Chemistry, 19.03.2020 00:48 yousifgorgees101
35.0 mL of stock hydrochloric acid solution is added to a 500 mL volumetric flask. A student adds distilled water up to the line. Using a pH meter and logarithms, he finds the molarity of the new solution to be 0.062 M HCl. What is the molarity of the stock solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

You know the right answer?
35.0 mL of stock hydrochloric acid solution is added to a 500 mL volumetric flask. A student adds di...
Questions in other subjects:

Business, 10.01.2020 03:31

History, 10.01.2020 03:31

Mathematics, 10.01.2020 03:31

History, 10.01.2020 03:31

French, 10.01.2020 03:31


History, 10.01.2020 03:31


History, 10.01.2020 03:31

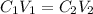
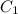 and
and 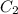 are initial and final concentration of a solution
are initial and final concentration of a solution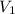 and
and 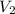 are initial and final volume of a solution
are initial and final volume of a solution ,
, 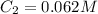 and
and 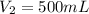
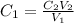 =
=  = 0.89 M
= 0.89 M

