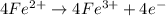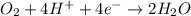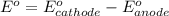Chemistry, 19.03.2020 00:52 gabischmid4340
A chemist designs a galvanic cell that uses these two half-reactions:
Half-reaction Standard reduction potential
O₂(g) + 4H⁺(aq) + 4e⁻ → 2H₂O(l) E⁰ red = +1.23 V
Fe³⁺(aq) + e⁻ → Fe²⁺(aq) E⁰ red = +0.771 V
(a) Write a balanced equation for the half-reaction that happens at the cathode.
(b) Write a balanced equation for the half-reaction that happens at the anode.
(c) Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written.
(d) Do you have enough information to calculate the cell voltage under standard conditions?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1

You know the right answer?
A chemist designs a galvanic cell that uses these two half-reactions:
Half-reaction Stan...
Half-reaction Stan...
Questions in other subjects:


History, 02.07.2020 03:01


Mathematics, 02.07.2020 03:01




Geography, 02.07.2020 03:01


Mathematics, 02.07.2020 03:01