Chemistry, 19.03.2020 00:53 zoeyandblaze
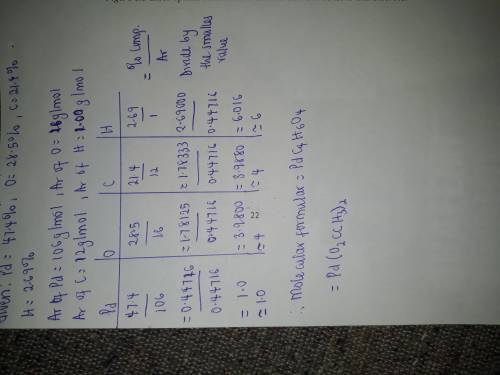
A compound has the percent composition 47.40% Pd, 28.50% O, 21.40% C, and 2.69% H. Based on this information, which molecular formulas could represent the compound? (Choose more than one answer)
PdO2C2H3
Pd(O2CCH3)2
Pd(O2C2H3)3
PdO4C2H9
Pd2C8H12O8

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, kajjumiaialome
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 22.06.2019 03:30, isabelvaldez123
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
A compound has the percent composition 47.40% Pd, 28.50% O, 21.40% C, and 2.69% H. Based on this inf...
Questions in other subjects:

History, 07.03.2021 08:10


English, 07.03.2021 08:10




Mathematics, 07.03.2021 08:10


Mathematics, 07.03.2021 08:10

History, 07.03.2021 08:10