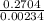Chemistry, 19.03.2020 00:28 itsmichaelhere1
Nitrogen reacts with chlorine to produce nitrogen trichloride. At equilibrium, the concentrations of each gas are as follows: 0.15 mol of nitrogen, 0.25 mol of chlorine, and 0.52 mol of nitrogen trichloride. Write the balanced equation for the reaction and calculate the equilibrium constant.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, BigDough9090
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2


Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Nitrogen reacts with chlorine to produce nitrogen trichloride. At equilibrium, the concentrations of...
Questions in other subjects:



Mathematics, 01.02.2021 17:50

Chemistry, 01.02.2021 17:50

Health, 01.02.2021 17:50

English, 01.02.2021 17:50



Social Studies, 01.02.2021 17:50

Mathematics, 01.02.2021 17:50

![\frac{[NCl_{3}^{2}]}{[N_{2}][Cl_{2}]^{3}}](/tpl/images/0552/9783/8a40e.png)
![\frac{[0.52]^{2}}{[0.15][0.25]^{3}}](/tpl/images/0552/9783/9ad1d.png)