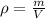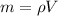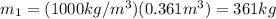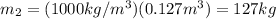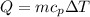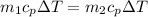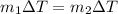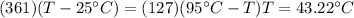Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1


Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
If you have 0.361 m 3 0.361 m3 of water at 25.0 ∘ C 25.0 ∘C and add 0.127 m 3 0.127 m3 of water at 9...
Questions in other subjects:



English, 05.02.2021 22:00






Mathematics, 05.02.2021 22:00

Arts, 05.02.2021 22:00