Chemistry, 19.03.2020 00:27 lashondrascott
Draw a mechanism for the protonation of water in sulfuric acid. Write out the structures of water and sulfuric acid completely, showing all bonds, electrons, and nonzero formal charges. Use curved arrow notation to show the reaction. Then draw the products of the reaction, again showing all bonds, electrons, and nonzero formal charges.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Draw a mechanism for the protonation of water in sulfuric acid. Write out the structures of water an...
Questions in other subjects:



Biology, 11.10.2020 16:01



Mathematics, 11.10.2020 16:01




Chemistry, 11.10.2020 16:01

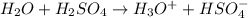 Mechanism has been shown below.
Mechanism has been shown below.