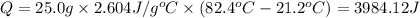Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1


Chemistry, 23.06.2019 06:40, Taylor73836
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
How many joules of heat are required to heat 25.0 g of isopropyl alcohol from the prevailing room te...
Questions in other subjects:





Mathematics, 19.05.2020 02:19

Business, 19.05.2020 02:19




Health, 19.05.2020 02:19

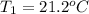
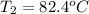
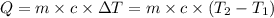
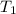 = Initial temperature of the substance
= Initial temperature of the substance 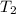 = Final temperature of the substance
= Final temperature of the substance