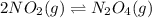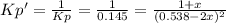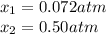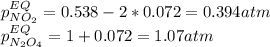Chemistry, 18.03.2020 21:59 briweaver9993
• Consider the reaction N2O4 (g) ⇌ 2 NO2 (g). At equilibrium, a 2.00-L reaction vessel contains NO2 at a pressure of 0.269 atm and N2O4 at a pressure of 0.500 atm. The reaction vessel is then compressed to 1.00 L. What will be the pressures of NO2 and N2O4 once equilibrium is re-established?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1


Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
• Consider the reaction N2O4 (g) ⇌ 2 NO2 (g). At equilibrium, a 2.00-L reaction vessel contains NO2...
Questions in other subjects:

Mathematics, 02.10.2019 21:20



Social Studies, 02.10.2019 21:20

Health, 02.10.2019 21:20


Physics, 02.10.2019 21:20



Social Studies, 02.10.2019 21:20

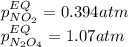
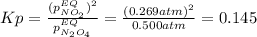
 is now present in order to take the reaction again to the equilibrium. Besides, the reaction changes as the products have more moles as:
is now present in order to take the reaction again to the equilibrium. Besides, the reaction changes as the products have more moles as: