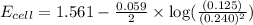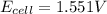Chemistry, 18.03.2020 21:41 cheerthi16
The standard reduction potentials for the Ag+|Ag(s) and Zn2+| Zn(s) half-cell reactions are +0.799 V and -0.762 V, respectively. Calculate the potential for the following electrochemical cell: Zn(s)|Zn2+(0.125 M)||Ag+(0.240 M)|Ag(s)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 07:30, apalacios3503
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
The standard reduction potentials for the Ag+|Ag(s) and Zn2+| Zn(s) half-cell reactions are +0.799 V...
Questions in other subjects:

Mathematics, 10.02.2021 20:50


Mathematics, 10.02.2021 20:50

History, 10.02.2021 20:50

Geography, 10.02.2021 20:50




Mathematics, 10.02.2021 20:50

Mathematics, 10.02.2021 20:50

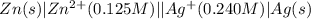
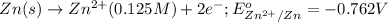
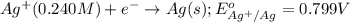 ( × 2)
( × 2)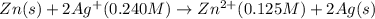
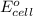 of the reaction, we use the equation:
of the reaction, we use the equation: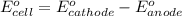
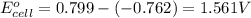
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Zn^{2+}]}{[Ag^{+}]^2}](/tpl/images/0552/5842/bffc2.png)
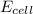 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[Zn^{2+}]=0.125M](/tpl/images/0552/5842/c12f1.png)
![[Ag^{+}]=0.240M](/tpl/images/0552/5842/c82de.png)