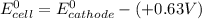A certain half-reaction has a standard reduction potential E⁰red = +0.63V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that must provide at least 1.30V of electrical power. The cell will operate under standard conditions.
(a) Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, write "no".
(b) Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, write "yes" and calculate the minimum. Round your answer to 2 decimal places. If there is no upper limit, write "no".

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, maymayrod2000
A32 year old immigrant from a patriarchal country is giving birth. as she is delivering the baby, she tearfully confesses to her doctor that this is her 4th child and she simply cannot handle any more children. she tells the doctor that her husband refuses to use contraception or allow her to, and she begs her doctor to tie her tubes and not tell her husband. the doctor complies. was hipaa violated? why or why not?
Answers: 3


Chemistry, 22.06.2019 08:00, katelyn0579
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely. analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2
You know the right answer?
A certain half-reaction has a standard reduction potential E⁰red = +0.63V. An engineer proposes usin...
Questions in other subjects:

English, 16.09.2021 14:00

English, 16.09.2021 14:00




Chemistry, 16.09.2021 14:00


Mathematics, 16.09.2021 14:00

Mathematics, 16.09.2021 14:00

Chemistry, 16.09.2021 14:00


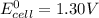
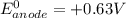
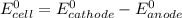
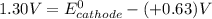
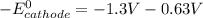
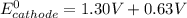
 ; then
; then 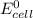 > 1.30 V
> 1.30 V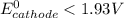 ; then
; then 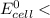 1.30 V
1.30 V