
Chemistry, 18.03.2020 18:30 justinb0829
Iron and vanadium both have the BCC crystal structure and V forms a substitutional solid solution for concentrations up to approximately 20 wt% V at room temperature. Compute the unit cell edge length for a 92 wt% Fe-8 wt% V alloy.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Iron and vanadium both have the BCC crystal structure and V forms a substitutional solid solution fo...
Questions in other subjects:

Mathematics, 16.09.2019 03:10

Mathematics, 16.09.2019 03:10


English, 16.09.2019 03:10


Biology, 16.09.2019 03:10

English, 16.09.2019 03:10


English, 16.09.2019 03:10

English, 16.09.2019 03:10

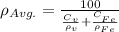
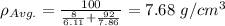
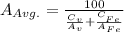
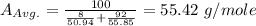
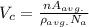
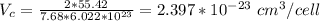
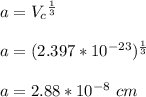 = 0.288 nm
= 0.288 nm

