Balance this reaction if necessary
Answer is C
NH3 + CO2 + H2O → NH4HCO3...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30


Mathematics, 05.02.2021 17:30


History, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30

Mathematics, 05.02.2021 17:30

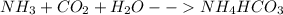
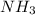 + mole of
+ mole of 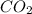 + 1 mole of
+ 1 mole of 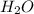 combines to give 1 mole of
combines to give 1 mole of 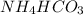 as a product.
as a product.

