
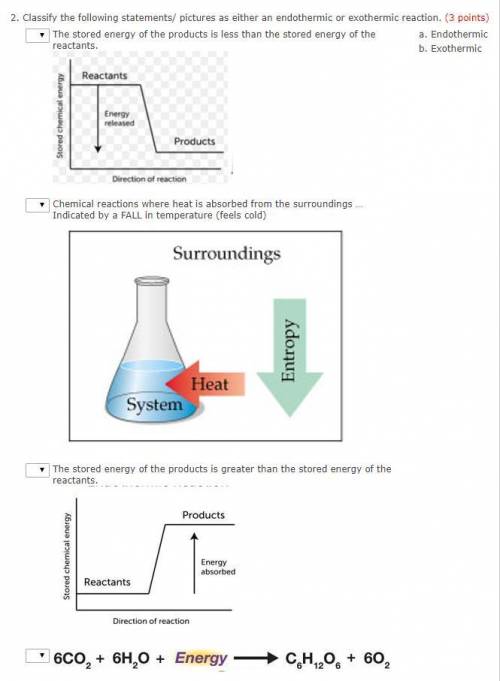
Classify the following statements/ pictures as either an endothermic or exothermic reaction. (3 points)
Chemical reactions where heat is absorbed from the surroundings … Indicated by a FALL in temperature (feels cold)
The stored energy of the products is greater than the stored energy of the reactants.
Chemical reactions where heat is released to the surroundings… Indicated by a RISE in temperature ( feels hot)
a. Endothermic
b. Exothermic

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3


You know the right answer?
Classify the following statements/ pictures as either an endothermic or exothermic reaction. (3 poin...
Questions in other subjects:

World Languages, 02.04.2020 14:53



Mathematics, 02.04.2020 14:55

Chemistry, 02.04.2020 14:55

Mathematics, 02.04.2020 14:56

Mathematics, 02.04.2020 14:56






