
Chemistry, 17.03.2020 20:00 davfar334p47luq
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially by acidifying the solution. Write all the relevant equilibrium equations and explain why adding acid will increase the solubility of calcium carbonate.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Calcium carbonate is an "insoluble salt". But the solubility of CaCO3 can be increased substantially...
Questions in other subjects:

Engineering, 03.09.2021 17:00

Mathematics, 03.09.2021 17:00





Physics, 03.09.2021 17:00



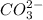 is converted to
is converted to 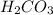 in acidic solution.
in acidic solution.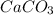 dissociates in solution to produce
dissociates in solution to produce 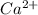 and
and 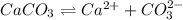
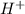 and gets converted to
and gets converted to 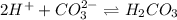 So,
So, 


