

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, danielahchf
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1
You know the right answer?
Calculate the mass of hydrogen formed when 27 g of aluminum reacts with excess hydrochloric acid acc...
Questions in other subjects:

History, 23.09.2019 01:00


Mathematics, 23.09.2019 01:00


History, 23.09.2019 01:00

Mathematics, 23.09.2019 01:00


Mathematics, 23.09.2019 01:00


 = 3 g of H₂.
= 3 g of H₂.
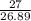
 =
= 


