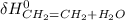Chemistry, 17.03.2020 19:23 alwayspouty6438
Industrial ethanol (CH3CH2OH) is produced by a catalytic reaction of ethylene (CH2═CH2) with water at high pressures and temperatures. Calculate ΔH o rxn for this gas-phase hydration of ethylene to ethanol, using bond energies and then using enthalpies of formation.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1


Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Industrial ethanol (CH3CH2OH) is produced by a catalytic reaction of ethylene (CH2═CH2) with water a...
Questions in other subjects:


Mathematics, 27.12.2019 10:31


English, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31

History, 27.12.2019 10:31


History, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31

Mathematics, 27.12.2019 10:31

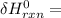 ∑ energy of old bond breaking + ∑ energies of the new bond formation.
∑ energy of old bond breaking + ∑ energies of the new bond formation.![[(4 * BE_{C-H}) + BE_{C-C}) + + BE_{O-H})]](/tpl/images/0550/7790/369dd.png) + ∑
+ ∑ ![[(5 * BE_{C-H}) + BE_{C-C}) + BE_{O-H}+ BE_{C-O})]](/tpl/images/0550/7790/f8d51.png)
![[4*413 kJ)+(614kJ)+(2*647kJ]](/tpl/images/0550/7790/b75fe.png) + ∑
+ ∑ ![(5*-413kJ)+(-347kJ)+(-467kJ)+(-358kJ)]](/tpl/images/0550/7790/da328.png)
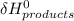 - ∑
- ∑ 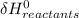
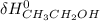 - ∑
- ∑