
Acetic acid has a Ka of 1.8*10^-5. Three acetic acid/ acetate buffer solutions, A, B, and C, wer made using varying concentrations: 1. [acetic acid] ten times greater than [acetate] 2. [acetate] ten times greater than [acetic acid] 3. [acetate] = [acetic acid] Match each buffer to the expected pH pH = 3.74 pH= 4.74 pH = 5.74

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
Acetic acid has a Ka of 1.8*10^-5. Three acetic acid/ acetate buffer solutions, A, B, and C, wer mad...
Questions in other subjects:




Chemistry, 10.11.2020 20:30


Physics, 10.11.2020 20:30




Mathematics, 10.11.2020 20:30

 for acetic acid is
for acetic acid is  . And, its
. And, its  value will be calculated as follows.
value will be calculated as follows.
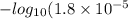
![pK_{a} + log \frac{[Salt]}{[Acid]}](/tpl/images/0550/7017/81f72.png)
![\frac{[\text{Acetate}]}{[\text{Acetic acid}]} = \frac{1}{10}](/tpl/images/0550/7017/c529e.png)
![pK_{a} + log \frac{[Acetate]}{[\text{Acetic Acid}]}](/tpl/images/0550/7017/a17de.png)

![\frac{[Acetate]}{\text{Acetic acid}}](/tpl/images/0550/7017/7d291.png) =
= 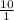

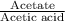 = 1
= 1



