Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, gomezyonathan93
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Consider the reaction: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O and ΔH = -2800 kJ. How many kJ of energy are rele...
Questions in other subjects:




Mathematics, 10.03.2021 04:00



Physics, 10.03.2021 04:00

English, 10.03.2021 04:00


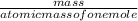
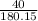
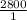 =
=