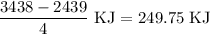Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

You know the right answer?
Find the ΔH for the reaction below, given the following reactions and subsequent ΔH values: PCl5(g)...
Questions in other subjects:




Mathematics, 19.01.2021 22:20




Mathematics, 19.01.2021 22:20


Advanced Placement (AP), 19.01.2021 22:20

 for the desired reaction will be 249.75 KJ.
for the desired reaction will be 249.75 KJ.