
Chemistry, 17.03.2020 05:59 milkshakegrande101
Balance the chemical equation given below, and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25°C? The density of nitrogen monoxide at 25°C is 1.23 g/L. ___ NH3(g) + ___ O2(g) → ___ NO(g) + ___ H2O(l)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1


Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

You know the right answer?
Balance the chemical equation given below, and calculate the volume of nitrogen monoxide gas produce...
Questions in other subjects:


Mathematics, 29.01.2021 19:30

Geography, 29.01.2021 19:30


Mathematics, 29.01.2021 19:30

Mathematics, 29.01.2021 19:30



Computers and Technology, 29.01.2021 19:30

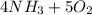 →
→ 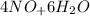
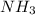 here acts as an limiting reagent.
here acts as an limiting reagent. 
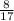 = 0.470 mol
= 0.470 mol
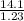 = 11.46 lit
= 11.46 lit 


