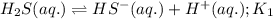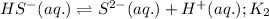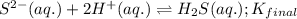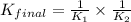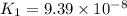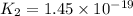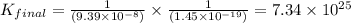Chemistry, 17.03.2020 04:33 channarlawassociate
Given the two reactions H2S(aq)⇌HS−(aq)+H+(aq), H2S(aq)⇌HS−(aq)+H+(aq), K1K1K_1 = 9.39×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), HS−(aq)⇌S2−(aq)+H+(aq), K2K2K_2 = 1.45×10−19, what is the equilibrium constant KfinalKfinalK_final for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, colochaortiz20p7cajw
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1



Chemistry, 22.06.2019 05:30, jameskarbar9p8c9d2
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1
You know the right answer?
Given the two reactions H2S(aq)⇌HS−(aq)+H+(aq), H2S(aq)⇌HS−(aq)+H+(aq), K1K1K_1 = 9.39×10−8, and HS−...
Questions in other subjects:



Health, 25.11.2021 05:30



Social Studies, 25.11.2021 05:30


Mathematics, 25.11.2021 05:30

English, 25.11.2021 05:30

Biology, 25.11.2021 05:30

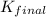 for the net reaction is
for the net reaction is