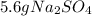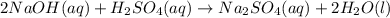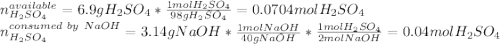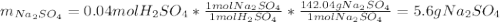Chemistry, 17.03.2020 04:36 GutsnGlory
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 6.9 g of sulfuric acid is mixed with 3.14 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 05:30, Dallas3506
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions in other subjects:

Geography, 26.11.2019 08:31

Chemistry, 26.11.2019 08:31



History, 26.11.2019 08:31


History, 26.11.2019 08:31



History, 26.11.2019 08:31