
Chemistry, 17.03.2020 02:23 miraclewhipppp
A 0.2722 g sample of a pure carbonate, X n CO 3 ( s ) , was dissolved in 50.0 mL of 0.1200 M HCl ( aq ) . The excess HCl ( aq ) was back titrated with 23.60 mL of 0.0980 M NaOH ( aq ) . How many moles of HCl react with the carbonate?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
A 0.2722 g sample of a pure carbonate, X n CO 3 ( s ) , was dissolved in 50.0 mL of 0.1200 M HCl ( a...
Questions in other subjects:





Mathematics, 03.06.2021 05:30

Mathematics, 03.06.2021 05:30



Mathematics, 03.06.2021 05:30

History, 03.06.2021 05:30

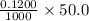 moles = 0.00600 moles
moles = 0.00600 moles
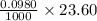 moles = 0.00231 moles
moles = 0.00231 moles

